Before the COVID-19 pandemic, the European Union (EU) was neither a strong promoter of global health nor a strong supporter of the World Health Organization (WHO). The Global Health Council Conclusions from 2010 were never comprehensively implemented and quickly forgotten. With the pandemic greatly affecting EU member states, the EU is increasingly interested in upholding multilateral cooperation in the global health field. Therefore, the EU should aim for an upgrading of the EU’s status in WHO, the establishment of a global health unit in the European External Action Service (EEAS), and an overhaul of the formal relationship between the European Commission and WHO.
The pandemic discloses the discrepancy between the EU advocating for global access to a COVID-19 vaccine while at the same time safeguarding its own access to it. Its refusal to alter patent laws that serve to protect the commercial and innovation interests of pharmaceutical companies based in EU countries can equally be questioned on grounds of global solidarity. A revamped global health strategy is needed to overcome such issues and make the EU a reliable and capable partner on global health that gives WHO a central role.
Global Health Policy Undervalued
As public health policy-making remains mainly a national competence under European legislation, the EU can coordinate and complement the policies of member states. The Union’s global health policy-making lacked visibility in recent decades, although the EU is traditionally a promoter of effective multilateralism. With its Council Conclusions on global health, adopted in 2010, the EU committed itself to stronger global health governance – including supporting WHO and the United Nations (UN) system – focusing on Universal Health Coverage, strengthening health systems, as well as recognising the need for a “Health in All Policies” approach, including in the EU’s external actions. However, the Conclusions never received the strong backing of health, development, and foreign ministries of EU member states, as the EU was primarily seen as a development actor rather than a strategic agent in global health. Thus, EU member states decided in an incoherent way on how large a budget that they and the European Commission would make available for international health priorities, initiatives, and institutions such as WHO. Before the COVID-19 pandemic, global health was not a priority on the European political agenda, and both the health and international development cooperation mandate was reclaimed by EU member states; with some exceptions being issues in fashion, such as anti-microbial resistance and digital health.
COVID-19: The EU’s Wake-up Call to Global Health?
The EU has been struggling to respond to the COVID-19 pandemic, as member states primarily followed a national response at the beginning. European and international cooperation were initially placed on the back burner with the introduction of export restrictions on protective equipment such as masks and gloves. Aside from the reluctance of member states to cooperate, the lack of resources and authority of the European Centre for Disease Prevention and Control (ECDC) has hampered a harmonised, evidence-based approach within Europe, and it has impeded the ECDC from proactively engaging in global health policies.
Gradually, a more “Europeanised” effort is now evolving to shore up the effectiveness of Europe’s public health response within the EU as well as in its multilateral commitments to bolster global health. European governments have started to realise that a joint approach is necessary to recover from the pandemic and the socio-economic crises that will follow. In her State of the Union address, Commission President Ursula von der Leyen called for a European Health Union. She announced plans to bolster the ECDC and the European Medicines Agency. An expansion of EU competence in the field of health is to be discussed in the Conference on the Future of Europe, which the European Commission will organise in 2021. She also announced the establishment of a European Biomedical Advanced Research and Development Authority (EU BARDA) to enhance Europe’s capacity to respond to cross-border threats.
Unfortunately, it is not clear if EU member states also support these ambitions. A proposal for the EU health budget (2021–2027) to be increased to 25 times its current size was largely undone by member states deciding to reduce the overall amount of the EU budget. A strong European investment in health systems and monitoring would have made global EU efforts in supporting the resilience of health systems and crisis preparedness more credible. Budgetary lines for global health policies for international cooperation have not been introduced or bolstered yet, which makes the future financing of ambitious EU global health policies in the upcoming EU budget challenging.
The Commission and EU member states were more united in February 2020, when they decided to uphold the international health order by activating financial support for WHO early on. During the pandemic, WHO has moved to the centre of information provision regarding the spread of the disease and the required public health responses. After harshly attacking WHO and accusing the organisation of being too China-friendly, the US administration announced in July 2020 that it would be pulling out of WHO. There are now increased expectations for the EU to fill financial as well as leadership gaps. EU member states such as Germany and France have already stepped in, with the former pledging an unprecedented €500 million to WHO for 2020. France has committed an additional €50 million to WHO as well as a €90 million commitment towards founding a new WHO Academy.
Formal EU and WHO Cooperation
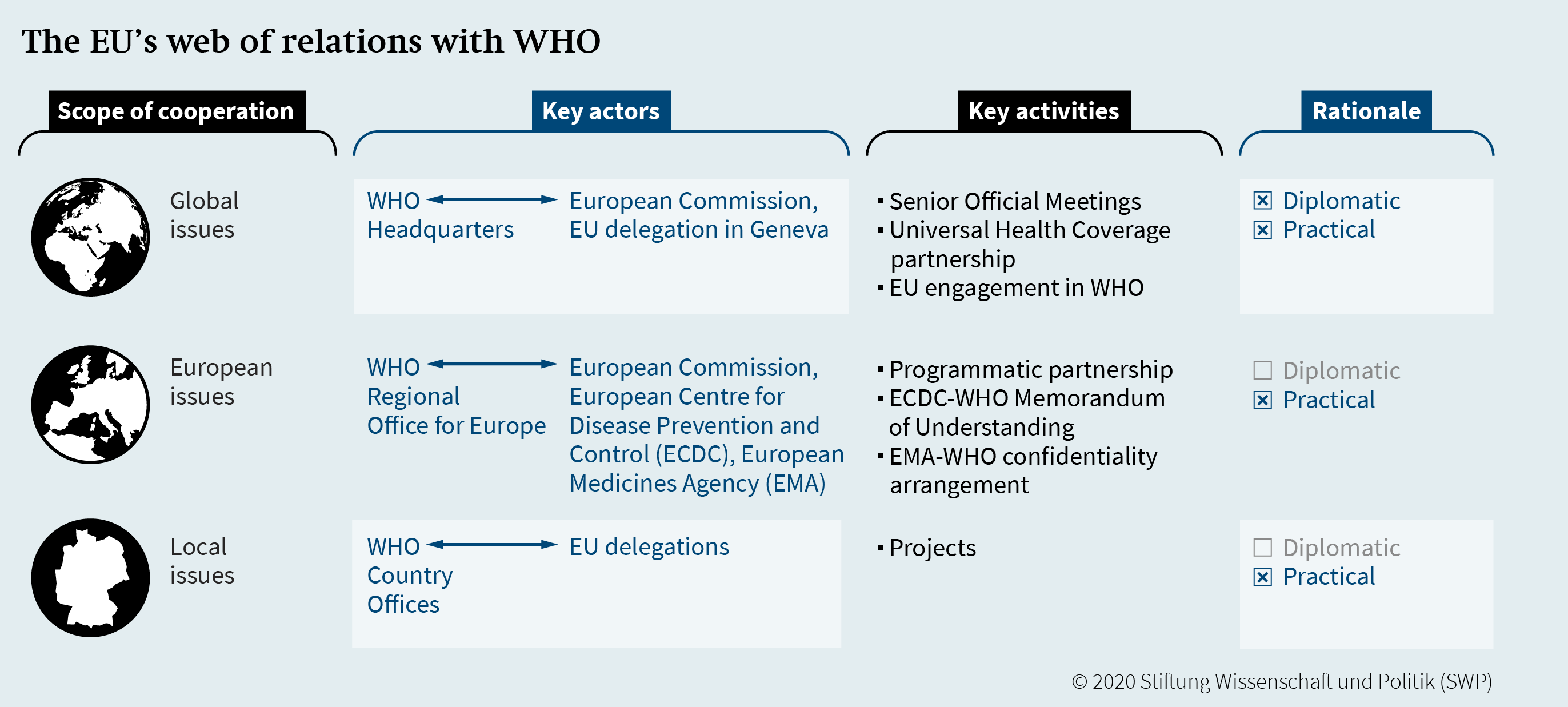
The relationship between WHO and the EU is based on an exchange of letters dating back to 1972. The EU–WHO cooperation is modelled on the work done by WHO and the EU on the global, regional, and national levels. Firstly, the EU and WHO Headquarters in Geneva interact through designated staff in the EU delegation and via Senior Official Meetings. Both are mostly concerned with global issues. Secondly, the European Commission as well as the ECDC have a practical partnership with the WHO Regional Office for Europe (WHO EURO) in Copenhagen, which is primarily focused on topics concerning the European region. Thirdly, the EU cooperates through its delegations with WHO country offices at the national level worldwide.
The coordination among EU member states on WHO matters has been prepared by the EU delegation in Geneva since 2010. Despite some initial questions on legitimacy and trust, it is now clearly in the driving seat to bring across a common EU position between European countries on key issues. It is backed by the European Commissions’ Directorate-General for Health and Food Safety (DG SANTE) and the EEAS. However, the EU only has an observer status, as only nation-states can join WHO. This prevents the Union from fully participating in WHO governing body meetings. Hitherto, the EU has not made any attempts to change this. However, with the current global climate of retreat from multilateralism, there might be a window of opportunity for the EU to upgrade its status as well as that of other regional economic integration organisations.
Despite various levels and areas of cooperation and the EU’s observer status in WHO’s governing bodies, the EU and WHO partnership still feels shaky and less clarified than it is for other partnerships between EU and UN institutions. The EU has, for instance, pushed for an enhanced observer status within the UN General Assembly (UNGA) that gives the Union, among others, the right to speak early in the debate of the UNGA and to be invited to the general debate. Furthermore, WHO is primarily considered a development organisation for public health standard-setting outside the EU. The COVID-19 pandemic may change this misconception for the better, since all countries are dependent on WHO recommendations, followed by many – but not all – EU member states.
The political support and increased joint action could strategically strengthen EU–WHO cooperation at all levels by building on existing collaboration and partnership models (Figure 1). Three aspects are critical in the EU’s web of relations with WHO. Firstly, the European Commission does not have formal partnerships with regional WHO offices aside from WHO EURO, which could enable the EU to engage in global health diplomacy within and outside the European region. Secondly, the cooperation with WHO EURO seems to be primarily focused on European issues, which is understandable. However, the next programmatic partnership between WHO EURO and the European Commission might therefore focus on global priorities that are equally important to both parties, such as projects about the environment and health, gender equity, and the commercial determinants of health. Thirdly, collaborative efforts between EU delegations with WHO country offices could be made more visible, coordinated, and harmonised through shared learning and training sessions.
The EU As a Geopolitical Actor in Global Health
Commission President von der Leyen has expressed a willingness of the Commission to become more geopolitical, which could imply a more proactive and instrumental approach to multilateral organisations, but it also bears the risk of implying an EU‑first bias. So far in the COVID-19 crisis, the EU has responded to the challenge of providing equitable access to vaccines, therapeutics, and diagnostics in three international fora.
Firstly, in early May 2020, the EU organised an international pledging conference to raise funds for the development of vaccines, therapeutics, and diagnostics. Later, a second conference was organised. These conferences can be regarded as a double-edged sword: On one side, they provide support for WHO’s goal to develop vaccines, therapeutics, and diagnostics as global public goods – goods that should benefit everyone equally. According to von der Leyen, the intention is not to distribute these exclusively among EU member states, but to make them available and affordable worldwide. On the other side, the conferences position the European Commission and the EU as leaders for COVID-19 solidarity, thereby sidelining WHO as the main platform for global coordination on international health priorities.
The EU pledging conferences are an example of “fast multilateralism”, but their focus is only on the development of vaccines, therapeutics, and diagnostics for one infectious pandemic disease, leaving other pressing health challenges neglected. Questions remain as to how more structural investment in and with WHO can be created to sustain global health multilateralism and create a sustainable impact on people’s health.
Secondly, in the first ever virtual World Health Assembly (WHA) – the highest decision-making forum of WHO’s member states – the EU led the development of the main resolution, which focused exclusively on the response to the COVID-19 outbreak. Multilateral support for this resolution came from China and the EU leadership, but not from Russia, the United States, or India – with the latter having a large pharmaceutical sector. The resolution includes four main features: the request for a broad UN response; a call to WHO member states to respect the International Health Regulations, the internationally binding set of rules to prevent, detect, and respond to infectious diseases; a call to international organisations to create a voluntary patent pool for the development of a COVID-19 vaccine to ensure affordable access for all; and the request for WHO to establish an impartial, independent, and comprehensive evaluation of the coordinated international health response to COVID-19.
The remuneration of pharmaceuticals is regulated by international patent law. However, since the global and simultaneous demand for COVID-19 diagnostics, vaccines, and therapeutics is so high, conventional patent licensing could make rapid development and large-scale production difficult, which therefore could delay access and distribution of a vaccine. According to the resolution, a COVID-19 technology access pool should be the mechanism to remedy this challenge, ideally based on best practices; one example is the UNITAID-established and supported Medicines Patent Pool.
However, the devil will be in the details, because the implementation of a patent pool requires internationally recognised Trade-Related Aspects of Intellectual Property Rights (TRIPS) flexibilities by the EU and its member states. These flexibilities are not discussed at WHO, but at the World Trade Organization TRIPS Council, where South Africa recently pushed for initiating a resolution with the aim of simplifying the requirements for TRIPS flexibilities, including compulsory licensing of COVID-19 diagnostics, therapeutics, and vaccines. This was proposed in order to legally guarantee access to diagnostics, therapeutics, and vaccines for COVID-19 as a global public good, including in low-income countries. The compulsory licensing of medical products from pharmaceutical and biotech companies can better protect public health and secure access to essential technologies. However, major pharma-producing countries, including from the EU, prioritise voluntary licensing and stress that the current market-based system suffices to guarantee access in low- and middle-income countries.
There seems to be a contradiction between the EU’s desire for global vaccine accessibility and EU member states’ commercial interests and political will to protect patents, since a lifting of patent restrictions could create a potential precedent for other vaccines and medicines. EU member states prefer to keep control over the licensing of new medical products, and therefore they opt for voluntary licensing via a patent pool. In theory, this could still allow global access, but the international experience with gaining access to medicines for other diseases, such as HIV/AIDS and hepatitis C, would indicate otherwise. The COVID-19 pandemic could potentially provide the momentum for reforming the governance of TRIPS flexibilities, which could have implications on whether universal access to medical products is allowed. The EU would benefit from this in the long term when considering both the economic and public health perspectives.
Thirdly, WHO and the European Commission co-host an “Access to COVID-19 Tools accelerator” Facilitation Council (COVAX facility), a new multi-stakeholder platform that is intended to guide key strategic, policy, and financial issues during the development of new COVID-19 diagnostics, therapeutics, and vaccines – with commitments by over 180 WHO member states. Still, parallel bilateral initiatives, such as advanced market commitments between the EU and pharmaceutical and biotech companies to secure doses of vaccines for European populations, might run against efforts within the COVAX facility to provide affordable vaccines for all, especially in low- and middle-income countries. However, the EU is now willing to engage in the COVAX facility after having advised its member states to not buy vaccines through COVAX earlier.
What is still missing is an outspoken stance on how WHO should function within the plethora of global health arrangements (World Bank, GAVI, Global Fund, etc.) – vis-à-vis other powerful stakeholders such as philanthropic institutes and the pharmaceutical industry – as an independent watchdog during infectious disease outbreaks (e.g. exposing cover-ups by states where an outbreak has started), as well as what its topics of focus should be and what organisational structure would be most adequate. In the lead-up to the announcement about the US withdrawal from WHO in July 2021, Germany and France allegedly were discussing WHO reform with the US administration, which points to a recognition of the need for changes to the current set-up. However, it is not clear which avenues of reform the European Commission and EU member states prefer. By intensifying cooperation with WHO, the European position on reform and the WHO reform process itself could be accelerated; despite WHO’s limitations, the pandemic has illustrated perhaps more than ever how much the organisation is needed. A non-paper presented by Germany and France gives some clues about the felt need for increased funding and a strengthening of the early warning and monitoring systems during epidemics and pandemics. But other issues, such as the regional structure of WHO and its norm-setting function as well as global health aid and advice to developing countries, were not addressed.
Future Choices for the EU on Global Health
As the COVID-19 pandemic enters a prolonged phase, the EU and its member states are in the position to jointly contain the virus and begin to structurally recover by investing in the development of strong and resilient public health systems. To become a reliable and capable partner for WHO and beyond, the EU could strengthen its capacities in the following areas.
Firstly, the EU could update its Council Conclusions on global health. A new, coherent EU global health strategy should focus on facilitating resilient health systems that are rooted in sustainable development as well as the right to health, in addition to being prepared for external shocks such as health security risks and consequences of climate change. A new global health strategy should offer a broad, more geopolitical, European perspective. Elements that could be included are references to the Union’s values (access to health, equality, democracy, accountability); links to the Sustainable Development Goals (SDGs); a health focus in all policies; a bolstering of the implementation of the International Health Regulations; as well as reference to the EU’s strategic autonomy with regard to medical supplies and medicines (see also Kickbusch and Franz).
New Council Conclusions should be accompanied by a concrete roadmap and monitoring mechanisms in order to be effective and transparent. Most important is that they be developed and owned by health, development, and foreign policy actors of the EU member states and institutions. Without their commitment, a recurrence of the 2010 Council Conclusions may happen when COVID-19 is behind us.
Secondly, the EU needs to establish strategic global health capacities within EU institutions and across different sectors – including trade, energy, and the European Semester of economic and fiscal policy coordination – followed by a clear mandate and solid financial global health resources. A strategic unit with financial, personnel, and thematic resources needs to be created within the EEAS that would have the mandate to coordinate several directorates on global health matters. One Commissioner should clearly be responsible on global health vis-à-vis the European Parliament, the European Council, and individual member states. This could either be the High Representative or the Health Commissioner. The unit in the EEAS would have to collaborate closely with experts from the Commissions’ DG SANTE and could liaise with WHO and other multilateral partners more strategically. Moreover, it could also have a specific global health diplomacy function as well as active collaboration with EU delegations contributing to its foreign policy.
Thirdly, the EU could strengthen its health competences domestically to be stronger abroad. Giving attention to, and linking, both the internal and external health dimensions of European policy, the EU could promote the internal strengthening of EU global and public health policy. The programme EU4Health 2021–2027, whose eventual budgetary allocation is still uncertain, should enhance European competences and coordination by boosting the EU’s preparedness for major cross-border health threats, strengthening health systems across the EU in an equitable way, as well as providing agreement on a common vaccine policy. To complement this, the ECDC could be strengthened and given a more prominent role and mandate in the EU’s global health policy-making. It is imperative for the EU to become more strategically autonomous with regard to medical supplies, but this should not be to the detriment of global solidarity.
Fourthly, the COVID-19 pandemic has also shown that EU member states have to act more coherently and in concert with EU institutions as well as during exchanges with civil society actors to avoid duplicating and contradicting (global) health policies. Therefore, a space for communication, coordination, and collaboration between EU institutions, EU member states, the European Parliament, and civil society actors has to be created in order to enhance the EU and member states’ abilities to perform more coherently on the international stage and within international partnerships, such as with WHO. The Global Health Policy Forum could be revived and upgraded for this purpose by broadening its functions as well as expanding membership to include the Council, the Parliament (aside from the Commission), the EEAS, and civil society actors.
Lastly, the EU needs to establish a strategic global health budget to pursue an ambitious agenda that is financially backed. The various budgetary channels that are supporting global health policies should be harmonised, or at least mapped. This would offer an overview of European financial resources for global health, making them transparent for the European public and helping with the strategic decision-making as to which partnerships should be financially supported, depending on the global health issue. Support for WHO could then be much more targeted and in coherence with other partnerships.
Recommendations
To strengthen and deepen its cooperation with WHO, the EU needs to increase its work in the following areas:
-
Upgrade the EU’s status at WHO: The European Commission and EU member states should jointly ask for an upgrading of the EU’s status with WHO to increase the EU’s visibility as a powerful unified actor and to enable it to speak with one voice. This could be done either through a resolution, a special agreement, or by strengthening WHO’s representation at the EU in Brussels, which is already working not only on a European but on a global mandate. In a first step, the EU could strengthen the partnership by solidifying the cooperation within a Memorandum of Understanding that replaces the exchanging of letters. More and well-coordinated meetings need to take place between senior representatives of WHO, the European Commission, and the EEAS. Consideration could be given to including representatives of EU member states to keep them engaged.
-
Extend the EU’s cooperation with WHO regional offices: A new roadmap for the partnership between WHO EURO and the European Commission is currently in the making. Now is the time for EU member states to have a strategic debate on WHO EURO and its future relations with the EU. New priorities and programmes should be aligned with achieving the SDGs – in Europe and globally. In line with the EU’s Green Deal objectives, projects with WHO promoting environment and health could equally pave the way for new areas of cooperation. A solid monitoring mechanism for the new five-year plan is key to creating a sustainable impact as well as accounting for joint actions. The establishment of formal relations with WHO regional offices outside of Europe, such as WHO AFRO, would put EU efforts at the country level within a broader synergistic and strategic approach.
-
Increase and sustain WHO’s budget: WHO’s financing is mainly based on individual donor interests, leaving WHO highly dependent and vulnerable to the top 15 donors, which contribute more than 80 per cent of all voluntary contributions. An increase of assessed and core voluntary contributions, as demanded by many experts as well as governments, is necessary to ensure WHO’s ability to act on its core functions. Financially, the announced US withdrawal could be partly compensated for by the EU, but the EU should also work for sustainable financing and reform of WHO, including ensuring autonomy and the global public legitimacy of the organisation. Sustainable and long-term predictable financing leads to sustainable human resources planning with staff that can implement reforms and deliver what is demanded of WHO.
-
Consider WHO recommendations and the results of the Independent Panel for Pandemic Preparedness and Response (IPPR): A high level of political support for WHO can be shown by applying WHO norms and standards at home as well as in international global arrangements. This should include unequivocal financial support by the EU and its member states for – as well as the commitment to – WHO’s COVAX facility. WHO’s role in global health can also be strengthened by referring to and promoting WHO’s role as the supreme global health authority. Based on the WHA resolution, WHO has established the IPPR, which evaluates the global COVID-19 response. This initiative is strongly supported by the EU and its member states and can, as an indirect effect, potentially defuse some of the geopolitical tensions around the global governance of the COVID-19 pandemic. The IPPR was launched in July 2020 and is co-chaired by former Prime Minister of New Zealand Helen Clark and former President of Liberia Ellen Johnson Sirleaf. An interim report to the WHA is expected in November 2020. European countries need to properly consider the results of the independent evaluation and further strengthen the autonomy of WHO.
-
Lead the WHO reform debates: The EU should have the ambition to reshape multilateral global health structures while establishing WHO at the centre. The EU should provide voice and leadership in an institutional and legitimate reform process of WHO, which was slow and ineffective before the COVID-19 pandemic. The German–French non-paper already provides relevant proposals.
-
Develop a new EU global health strategy that addresses WHO reform and is backed by health, development, and foreign affairs stakeholders from EU institutions and member states. Such a global health strategy should include issues regarding WHO’s raison d’être, its current organisational structure, areas of focus, and independence during outbreaks of infectious diseases. It should also make choices about, or create a balance between, the EU’s desire to uphold multilateral arrangements and simultaneously become more strategically autonomous.
A renewed partnership between the EU and WHO during the COVID-19 pandemic – despite nationalistic trends and geopolitical tensions – offers a glimmer of hope. The EU should seize on this opportunity but not outshine WHO, as collective efforts are needed more than ever to secure global public goods and uphold the international health order.
Susan Bergner and Maike Voss are Associates in the Global Issues Division at SWP. Both work in the “Global Health” project, which is funded by the German Federal Ministry for Economic Cooperation and Development.
Remco van de Pas is a public health doctor and global health researcher. He is a Research Fellow at the Institute of Tropical Medicine, Antwerp, and Research Associate at the Clingendael Institute.
Louise van Schaik is Head of Unit EU & Global Affairs at the Clingendael Institute.
© Stiftung Wissenschaft und Politik, 2020
All rights reserved
This Comment reflects the authors’ views.
SWP Comments are subject to internal peer review, fact-checking and copy-editing. For further information on our quality control procedures, please visit the SWP website: https://www.swp-berlin.org/en/about-swp/ quality-management-for-swp-publications/
SWP
Stiftung Wissenschaft und Politik
German Institute for International and Security Affairs
Ludwigkirchplatz 3–4
10719 Berlin
Telephone +49 30 880 07-0
Fax +49 30 880 07-100
www.swp-berlin.org
swp@swp-berlin.org
ISSN 1861-1761
doi: 10.18449/2020C47