The EU’s Open Strategic Autonomy in the Field of Pharmaceuticals
Overcoming import dependencies for antibiotics through the EU authority HERA
SWP Comment 2023/C 02, 11.01.2023, 8 Pagesdoi:10.18449/2023C02
Research AreasThe COVID-19 pandemic and war in Ukraine have highlighted the dependence of the European Union (EU) on individual trading partners. One of the tasks of the European Commission’s new Directorate-General, the Health Emergency Preparedness and Response Authority (HERA), established in 2021, will therefore be to contribute to the EU’s “open strategic autonomy” by identifying and eliminating import dependencies in the field of pharmaceuticals. HERA’s work thus aligns with current EU efforts to reduce concentrated import risks. Here, three aspects of this work are particularly important: the identification of dependencies, the development of strategies to overcome them and the incorporation of these strategies within global health governance.
In March 2020, the United Nations (UN) and other organisations and countries called for solidarity in the fight against COVID-19. In the same month, however, many countries introduced restrictions on the export of healthcare products. India, for example, limited its exportation of medicines such as antibiotics and paracetamol. China and the USA limited their exportation of personal protective equipment (PPE) and respirators. Aside from using such restrictions to simply protect their own populations, some countries employed these controls as geopolitical tools. In this context, the EU and member states such as Germany introduced policies that required preapproval before PPE could be exported – even within the EU.
Trade restrictions are not uncommon under the World Trade Organization (WTO) regime. The trade of health goods, however, is of particular significance, as supply shortages and price increases in this market can strain even the most stable health systems and pose a serious threat to the global South’s supply of these goods. In addition, restrictions on the trade of health commodities threaten the EU’s internal market because states may feel compelled to take unilateral action to protect their own populations. The COVID-19 pandemic has shown that this can occur. HERA is therefore designed to counteract these developments and crisis-proof the EU’s supply of pivotal health goods.
The establishment of HERA
The pandemic has shown that coordinated action at the European level is necessary to counter public health crises. In view of the trade dependencies that have become apparent and the fragility of supply chains, a central task of HERA will be to secure the European population’s supply of healthcare goods.
The establishment of HERA is based on a decision issued by the European Commission on September 16, 2021. Institutionally, HERA is set up as a Directorate-General and reports to the European Commissioner for Health and Food Safety. It is thus less autonomous from the EU Commission than an Agency. In essence, HERA is intended to ensure member states’ unified and coordinated approach in two “working modes”, namely preparedness and crisis response. Although member states are directly involved in the execution of tasks by way of their inclusion in the “HERA Board” and the “HERA Advisory Forum”, this constitutes their only direct influence over the Authority, since the establishment and budget of Directorates-General are carried out by the Commission. This creates tension, as member states’ health policies and health care regimes are exempt from harmonisation by Article 168(5) of the Treaty on the Functioning of the EU (TFEU). Although HERA’s mandate is not focused on harmonisation, its establishment as an Agency as opposed to a Directorate-General could have reduced member states’ concerns and created more transparency vis-à-vis the European Parliament (EP) and the Council of the European Union.
HERA has five tasks nested under the preparedness “working mode”: (1) monitoring and assessing potential threats and developing countermeasures, (2) promoting research, (3) advancing knowledge and skills in preparedness and response, (4) procuring, stockpiling, and distributing medical supplies, and (5) “addressing market challenges” and strengthening “open strategic autonomy”.
The budget to fulfil these tasks is € 6 billion over six years. Comparatively, the Biomedical Advanced Research and Development Authority (BARDA) in the US had a $1.6 billion working budget in 2022, therefore, it would seem that HERA’s funding is at a similar level in terms of GDP although the US has a smaller population than the EU and BARDA has less tasks. HERA’s funding is provided through the Multiannual Financial Framework 2022–2027 and NextGenerationEU. In 2022, € 165.3 million have been allocated to strengthening “open strategic autonomy” in the field of health commodity manufacturing.
HERA’s mission aligns with the EU’s overall trade policy objective of achieving more “open strategic autonomy” as formulated in the Trade Policy Review. This desired “open strategic autonomy” is defined as the ability to make decisions independently while taking into account one’s own interests and values. The word “open” indicates that free and fair trade with partners is not decoupled from the approach, but always in the foreground. In the health sector, similar conflicting objectives and problems arise to those that emerge in other areas where supply chains are currently restructured. This dynamic will be analysed below through the lens of the EU’s quest to identify dependencies in the trade of healthcare goods via HERA.
The identification of dependencies
A key part of HERA’s work will initially be to identify import dependencies. As early as 2020, the EP highlighted the importance of the geopolitical dimension of drug imports, as China and India, for example, produce 60 to 80 per cent of active pharmaceutical ingredients (APIs). In addition, according to the German Federal Institute for Drugs and Medical Devices (BfArM), there are already significant supply shortages of active ingredients in Germany.
While all medical goods are of interest in this context, a special focus should be placed on antibiotic APIs. This is because antibiotic APIs can currently hardly be produced at cost-covering levels in the EU, which is why there are few incentives to reshore their production. The problem therefore lies less in the creation of production capacities, but rather in the fact that investments in this sector are unprofitable due to high labour costs and low (fixed) prices. This reality is compounded by supply shortages and research and development needs resultant of growing antimicrobial resistances (AMR). This is particularly problematic as antibiotics are used at all stages of medical treatment, whether conservative or surgical.
According to UN Comtrade data, the volume of EU antibiotic imports has decreased slightly since 2001. This is also in line with the findings of the European Centre for Disease Prevention and Control (ECDC), according to which the use of antibiotics in human medicine has been declining in the EU. The same also applies to the import surplus, which has been in decline.
Still, the data nonetheless indicates that the EU is dependent on imports for its antibiotics. To determine whether these dependencies are concentrated risks that have the potential to limit the EU’s open strategic autonomy, the relevant trading partners must be identified. Important in this analysis is the distinction between finished pharmaceutical products (FPPs) and APIs. The following analysis excludes FPPs and only looks at APIs, as these are the key element for the production of antibiotics in the EU.
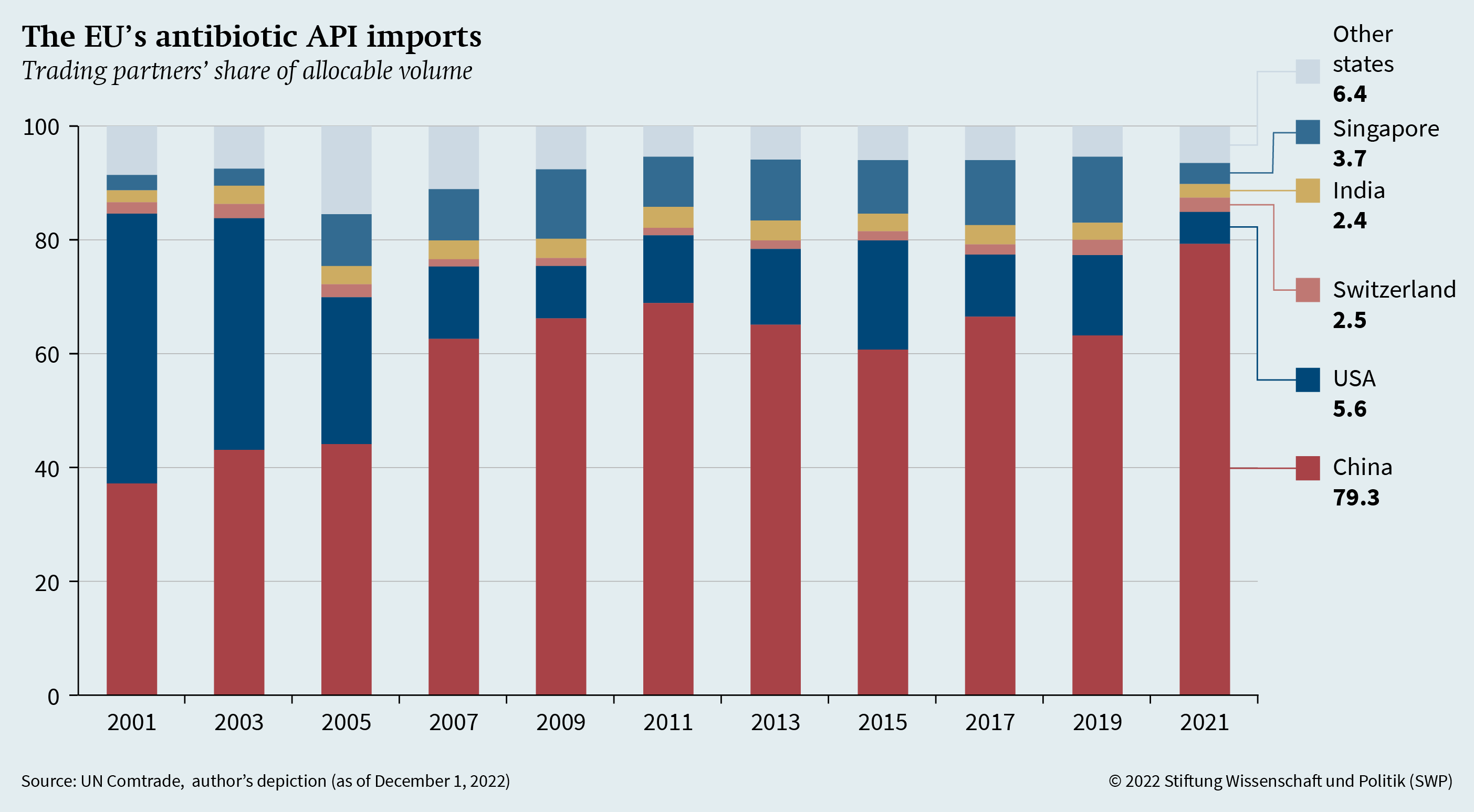
Ensuring open strategic autonomy in the field of antibiotics first requires the identification of dependencies in antibiotic API imports. Figure 1 (see p. 4) shows the total volume of antibiotic API imports (for human and veterinary use, HS 2941) distributed among the EU’s five largest trading partners in 2021, since 2001. Replication materials for Figures 1 and 2 are available at https://doi.org/10.7802/2492. A remainder that cannot be attributed to any partners is not included. This figure clearly shows the EU’s increasing dependency on Chinese antibiotic APIs. While about 37 per cent of all EU API imports came from China in 2001, this share more than doubled to about 79 per cent in 2021. Even if the unallocated remainder of imports is taken into account, China’s overall share of all EU API imports is still around 66 per cent. The US, on the other hand, lags far behind in second place, accounting for only about 6 per cent. Twenty years ago, the US was the EU’s largest API trading partner, accounting for around 48 per cent of its API imports. Thus, there is an extraordinarily strong import concentration of Chinese APIs.
Figure 1 also underscores the need to look at the actual weight of imports rather than just their value; because in reality, based on value alone, China would only account for about 28 per cent of EU API imports in 2021. Looking at the value thus leads to a representation that is distorted by price differences, thereby masking dependencies. Similarly, dependencies are underestimated when imports of antibiotic FPPs are considered: Here, China is still the most important import partner, but it accounts for only about 24 per cent of the EU’s overall FPP imports.
Pathways to open strategic autonomy in antibiotics
In line with HERA’s mission, the Commission’s Communication on the Pharmaceutical Strategy explicitly references “open strategic autonomy”. Further, the Commission identifies three paths to reducing dependencies, namely by (1) producing in Europe, (2) diversifying value and supply chains, and (3) stockpiling.
Still, it is largely unclear which exact measures HERA will take in order to achieve the goals stated in the pharmaceutical strategy. Therefore, the following sections outline how the path to open strategic autonomy could be realised in the field of antibiotics by looking at initiatives in other areas.
![]() Building European capacity
Building European capacity
In early 2022, the Commission initiated a project in the field of semiconductor technology with the European Chips Act, focusing on the development of European capacities. The Commission’s proposal to build European capacity in the field revolves around three “pillars”, and construction of the first production facilities are already underway. The provisions of this Act could also be applied to pharmaceutical production.
The first pillar creates a framework for increasing production capacities. To this end, funds from the InvestEU Fund are made available primarily to support small and medium-sized enterprises (SMEs) and attract investors.
The funds available to HERA could be used in a similar way to create analogous incentives in pharmaceutical manufacturing so that the few remaining production sites in the EU remain here; in the same vein, these funds could be used to help bring production back to the EU from abroad (reshoring). Since profitability is a key problem, production must be supported financially and/or price increases must be compensated in such a way that makes it lucrative to produce antibiotic APIs while simultaneously ensuring that FPPs remain affordable. Links can be made here to the Important Project of Common European Interest (IPCEI) in the health sector, which provides a common regulatory framework for financing innovative industrial activities.
In addition to the fundamental problems arising from the restructuring of supply and value chains, reshoring production to Europe is above all associated with high costs. Any initiative to reshore production must therefore resolve the tension that exists between (fixed) prices for pharmaceuticals, the imperative to ensure their affordability and the higher production costs in Europe. This requires government intervention in the market. The effects of reshoring and increasing production must also be closely monitored because spill-over effects could jeopardise supply in other non-EU countries.
In order to attract investment, it is also necessary to ensure long-term security through agreements. In addition, investments will need to be screened to avoid creating new dependencies on foreign investment. Moreover, it should be considered whether companies can be promised fixed purchase quotas for the antibiotic FPPs they produce, which would then flow into the stockpiling strategy or World Health Organization (WHO) aid programs.
The second pillar of the European Chips Act is a coordination mechanism, which among other things monitors shortages and bottlenecks. While there is still no equivalent of the first pillar in the field of medicines, the range of tasks of the European Medicines Agency (EMA) has already been expanded to include monitoring supply shortages. To this end, EMA set up an Executive Steering Group on Shortages and Safety of Medicinal Products (MSSG), which draws up a list of critical medicines, monitors their supply and demand, and submits reports and recommendations on how to combat shortages. This division of labour will require intense collaboration between EMA and HERA.
The third and final pillar of the European Chips Act centres on public-private partnerships and blended funding in launching pilot facilities and centres of excellence. In view of increasing antimicrobial resistance, it is advisable to continue to strengthen research and development as a component of extending the EU’s open strategic autonomy. Public-private partnerships and funding structures could provide incentives to stimulate the less lucrative development of new active substances and reduce investment risks. To avoid parallel structures, cooperation with the Innovative Health Initiative (IHI), the Incubator for Antibacterial Therapies in Europe (INCATE) initiative, and with the Global Partnership for Research and Development of New Antibiotics (GARDP), which is also already supported by the German Federal Ministry of Health (BMG), is advised.
Diversifying trade
The relocation of production to the EU can succeed, but only if enormous price pressures allow it. Therefore, it is crucially important that the EU diversifies its trade. Here, too, it is worth taking a look at other EU initiatives, the most recent and probably most relevant of which are the planned Critical Raw Materials Act (CRMA) and the already existent European Raw Materials Alliance (ERMA).
ERMA is an EU-subsidised global network of companies, investors, research institutions, government agencies and organisations. One goal of the Alliance is to stabilise and diversify supply chains by establishing investment channels. These channels are established between European and non-European companies by identifying investment opportunities and bringing together capital providers and other enterprises through a Raw Materials Investment Platform (RMIP).
Applied to the production of antibiotics, this would mean establishing a platform with financial support from the EU that brings stakeholders together and opens up new supply and value chains. Such a platform could be used to find new suppliers in third countries to produce raw materials needed in antibiotics; it could also work to stabilise value and supply chains with the help of investments, similar to the Minerals Security Partnership (MSP). A more extensive option for the platform would be for it to directly support capacity building efforts in third countries – where antibiotics may also be needed.
In connection with the diversification of supply and value chains, there is increasing discussion of “friend-shoring”, i.e., relocating production to friendly or trusted countries. While this approach can increase supply security and inspired the foundation of the MSP, it must be kept in mind that political climates can change rapidly and supply chains are not amenable to constant dynamic adjustment. Therefore, fundamental diversification is still needed.
With regard to the area of critical raw materials, a regularly updated list of critical medicines could be maintained, equivalent to the Critical Raw Materials List published every three years. In doing so, not only could shortages be assessed but also market concentrations could be revealed and generic substitution taken into account. This initiative could be linked to the list of critical medicines for COVID-19 that is already compiled by EMA or the lists on scarce goods resultant of supply bottlenecks caused by production shortages.
Stockpiling goods
The third and final component of the strategy that HERA will play a role in implementing is the stockpiling of commodities. Because of their long shelf life, antibiotic FPPs are particularly suitable for stockpiling. However, the inefficiency risk associated with stockpiling costs and waste (because of expiration) must always be kept in mind. Taking these risks into account, parallels could be drawn to already existent stockpiling strategies and initiatives that could be expanded to include antibiotic storage. A direct starting point is the EU Civil Protection Mechanism and its associated reserves for disaster situations (rescEU). During the pandemic, rescEU was used to build up and make available stocks of medical equipment within the EU.
Currently, mainly medical masks, gloves and protective kits, but also several thousand respirators, are stocked. These reserves are currently housed in nine EU member states. In terms of storage and logistics, stockpiling is entirely the responsibility of the EU Commission; the member states are only responsible for procurement. These procedures from the Civil Protection Mechanism could easily be extended to include antibiotics, which could be procured by member states, stored in a decentralised manner and distributed during shortages. However, it would be advisable to decouple these reserves from typical disaster situations that occur in the form of disease outbreaks. Rather, the lack of antibiotics due to the collapse of supply chains or geopolitical crises should be classified as a disaster case in itself, thereby triggering the mechanism.
In addition to synergising with the Civil Protection Mechanism, the institutionalised stockpiling of pharmaceuticals would offer the possibility to quickly move antibiotics to third countries when disaster strikes. This would also open the door to incorporating EU pharmaceutical strategies in global health governance.
Incorporation into global health governance
While the EU’s pursuit of greater strategic autonomy is indispensable in safeguarding its values and interests, it must be careful not to undermine them at the same time. As a civil and normative power, the EU should not decouple from international partners in global health governance; doing so would be incompatible with its self-image and stated goal of achieving “open” strategic autonomy. Rather, its pursuit of this aim should only be seen as a means of overcoming concentrated import risks, and the EU should embed this aspiration in its overall geopolitical strategy, especially with regard to the EU’s relations with China.
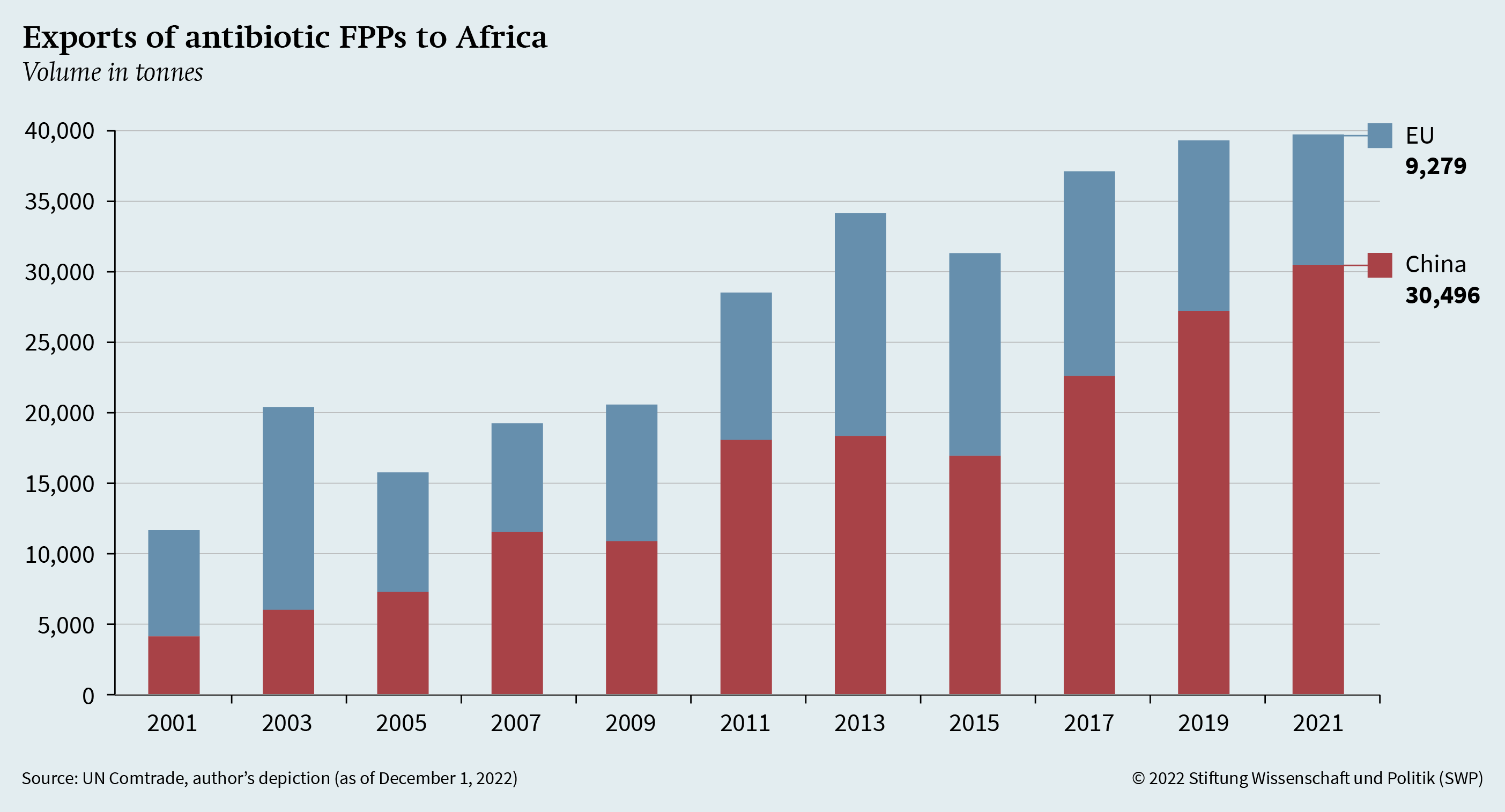
Given China’s growing influence in Asia and Africa, the EU has already begun to strengthen ties with the African continent, most recently launching a € 300 billion investment plan under the Global Gateway Initiative. While strategic competition with China in Africa is particularly evident in the areas of infrastructure and lending, there are also increasing linkages between African countries and China in the health sector. These relate to disease surveillance and treatment and the supply of health commodities, such as antibiotic FPPs.
To shed light on these linkages, Figure 2 shows the export volume of antibiotic FPPs (HS 300410 and 300420) from China and the EU to African countries. While the EU’s volume has been relatively stable with minor fluctuations, Chinese exports have increased significantly since 2001. Therefore, in view of EU geopolitical strategies and HERA’s work, the dependencies of third countries must also be taken into account. In addition, the dependencies of other countries in the global South outside of Africa need to be examined.
In principle, there are two ways in which HERA’s sought after diversification of antibiotic supply can be incorporated into global health governance: (1) by collaborating with third countries where antibiotics may be needed, and (2) by integrating HERA’s activities into WHO programs.
Although it would be difficult to fully produce antibiotics in third countries that would otherwise receive antibiotic FPPs by way of development cooperation, these countries could still be included in supply and value chains. This will also require expanding the WTO Pharma Agreement. It would also be possible to involve the European and Developing Countries Clinical Trials Partnership (EDCTP) here.
In addition, it would make sense to intensify cooperation with the WHO in the context of building European production capacities and the planned stockpiling of medical goods in the EU. For example, EU purchase quotas for antibiotics produced within Europe could be an instrument to increase the attractiveness of producing in the EU, whereby the antibiotics acquired in this way could then be shipped to third countries within the framework of WHO programs or in the event of a disaster within the framework of rescEU. This would give the EU the opportunity to expand its role in global health as an “emergency pharmacy”.
Conclusion and recommendations
The EU’s newly created authority HERA is taking a central role in reforming the European health architecture. One goal of this reform is to increase the EU’s open strategic autonomy in the field of pharmaceuticals. While this aim is relatively clearly stated, the path to achieving it is hardly spelled out. The current revision of the EU’s general pharmaceutical legislation, joint procurement of vaccines and plans to establish incentive structures for research into new antibiotics, are just the beginning of this journey. In view of seizing opportunities and developing strategies in this realm, the EU should:
-
Identify dependencies: Concentrated import dependencies in the health sector must be comprehensively analysed. The EU’s strong import dependence on China in the area of antibiotic APIs has already been identified. Further, analyses must not only focus on the value of imports, but also on their quantity. Likewise, a distinction must be made between APIs and FPPs. In addition, individual components for production must also be considered, such as nutrients.
-
Pursue the paths to autonomy: Capacity building, import diversification and stockpiling appear to be suitable measures to reduce the EU’s strategic dependencies. When it comes to the concrete design of these measures, it is advisable to adopt action from similar initiatives. The European Chips Act, the Critical Raw Materials Act, the European Raw Materials Alliance and the EU Civil Protection Mechanism are particularly suitable.
-
Incorporate aims into global health governance: In the pursuit of strategic autonomy, the EU runs the risk of quickly decoupling from partners and processes in global health governance. This can be avoided by building value and supply chains with third countries, many of which are currently dependent on China, and by embedding the EU’s new capacity and supplies into assistance programs.
In addition to pursuing these objectives, the German government should, first, move away from the current trade model in the pharmaceutical sector, in particular by relocating production to the EU and by diversifying pharmaceutical trade. For geopolitical reasons, the existing model of importing low-priced pharmaceuticals from individual countries acting as “pharmacies of the world” must be overcome as quickly as possible. The law announced by the BMG intends to surmount supply shortages and is an important step in the right direction. However, it will be necessary to integrate it into EU strategies. To this end, Germany’s future budget contributions to the IPCEI should also be increased. Second, the German government must develop a way to deal with rising prices. The diversification of trade and supply chains will bring with it considerable costs, and the resultant rise in prices must be adequately communicated and comprehensively cushioned.
Michael Bayerlein is an Associate in the EU/Europe Research Division at SWP, where he works on the project “Global and European Health Governance in Crisis” funded by the Germany Federal Ministry of Health (BMG).
© Stiftung Wissenschaft und Politik, 2023
All rights reserved
This Comment reflects the author’s views.
SWP Comments are subject to internal peer review, fact-checking and copy-editing. For further information on our quality control procedures, please visit the SWP website: https://www.swp-berlin.org/en/about-swp/ quality-management-for-swp-publications/
SWP
Stiftung Wissenschaft und Politik
German Institute for International and Security Affairs
Ludwigkirchplatz 3–4
10719 Berlin
Telephone +49 30 880 07-0
Fax +49 30 880 07-100
www.swp-berlin.org
swp@swp-berlin.org
ISSN (Print) 1861-1761
ISSN (Online) 2747-5107
DOI: 10.18449/2023C02
(English version of SWP‑Aktuell 75/2022)